Ideje 31+ Structure Of Atom Of Fluorine Čerstvé
Ideje 31+ Structure Of Atom Of Fluorine Čerstvé. Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. Then play a game to test your ideas!
Nejchladnější Fluorine Protons Neutrons Electrons Electron Configuration
Structure can be written as part of o chemlcai symbol. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride. Fluorine is a chemical element with the symbol f and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom.The chemical symbol for fluorine is f.
Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride. Then play a game to test your ideas! Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas.

Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. These atoms differ in the number of neutrons.

Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Basic atomic structure worksheet and the 1. The number of protons in one atom of. These atoms differ in the number of neutrons. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride.. The nucleus is composed of protons and neutrons.

The chemical symbol for fluorine is f.. These atoms differ in the number of neutrons. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms... Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride.
Then play a game to test your ideas! Then play a game to test your ideas! Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. The chemical symbol for fluorine is f. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride. Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. The average atomlc mass is the weighted average of all the isotopes of an element. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. As an example, an oxygen atom has six electrons in its outer shell.

Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.. . In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons.

The nucleus is composed of protons and neutrons.. Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Structure can be written as part of o chemlcai symbol. The average atomlc mass is the weighted average of all the isotopes of an element. Then play a game to test your ideas! As an example, an oxygen atom has six electrons in its outer shell. Among the elements, fluorine ranks 24th in universal abundance and 13th in. The 3 particles of the atom are:

Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine. The number of protons in one atom of. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. As an example, an oxygen atom has six electrons in its outer shell. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine. The nucleus is composed of protons and neutrons. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Then play a game to test your ideas! Structure can be written as part of o chemlcai symbol. Basic atomic structure worksheet and the 1. Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. Then play a game to test your ideas!

Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it. Among the elements, fluorine ranks 24th in universal abundance and 13th in. The nucleus is composed of protons and neutrons. The number of protons in one atom of.. Fluorine is a chemical element with the symbol f and atomic number 9.

Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell.. Basic atomic structure worksheet and the 1. Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. Fluorine is a chemical element with the symbol f and atomic number 9. In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons. These atoms differ in the number of neutrons. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it.

In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons... The 3 particles of the atom are: As an example, an oxygen atom has six electrons in its outer shell. Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure.

Fluorine is a chemical element with the symbol f and atomic number 9. Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell... Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms.

In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons... The 3 particles of the atom are: Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Basic atomic structure worksheet and the 1. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. These atoms differ in the number of neutrons. Fluorine is a chemical element with the symbol f and atomic number 9. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it.

The number of protons in one atom of.. The average atomlc mass is the weighted average of all the isotopes of an element. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it.

Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms.. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure.. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms. As an example, an oxygen atom has six electrons in its outer shell. The number of protons in one atom of. Basic atomic structure worksheet and the 1. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Structure can be written as part of o chemlcai symbol. The 3 particles of the atom are:. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial.

As an example, an oxygen atom has six electrons in its outer shell. Fluorine is a chemical element with the symbol f and atomic number 9. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. Basic atomic structure worksheet and the 1. Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. The 3 particles of the atom are:

The number of protons in one atom of.. As an example, an oxygen atom has six electrons in its outer shell. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine.

In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it.. Among the elements, fluorine ranks 24th in universal abundance and 13th in.. The 3 particles of the atom are:

Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses.. . These atoms differ in the number of neutrons.

Basic atomic structure worksheet and the 1... The number of protons in one atom of. The nucleus is composed of protons and neutrons. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms... In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons.

Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The 3 particles of the atom are: These atoms differ in the number of neutrons. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it.. Among the elements, fluorine ranks 24th in universal abundance and 13th in.

These atoms differ in the number of neutrons. . In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it.

The 3 particles of the atom are: Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Then play a game to test your ideas! The chemical symbol for fluorine is f. As an example, an oxygen atom has six electrons in its outer shell. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas.

As an example, an oxygen atom has six electrons in its outer shell... Fluorine is a chemical element with the symbol f and atomic number 9. The number of protons in one atom of. The chemical symbol for fluorine is f. The 3 particles of the atom are: Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses.. Basic atomic structure worksheet and the 1.

Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms. Nov 03, 2021 · the formal charge on the fluorine atom, f = 7 −12(2) − 6 = 0. The number of protons in one atom of. The average atomlc mass is the weighted average of all the isotopes of an element. The nucleus is composed of protons and neutrons. Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom... Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell.

Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. The 3 particles of the atom are:.. Among the elements, fluorine ranks 24th in universal abundance and 13th in.

Structure can be written as part of o chemlcai symbol.. As an example, an oxygen atom has six electrons in its outer shell. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms. The chemical symbol for fluorine is f. Then play a game to test your ideas!.. Basic atomic structure worksheet and the 1.

Fluorine is a chemical element with the symbol f and atomic number 9... Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine. These atoms differ in the number of neutrons.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

Among the elements, fluorine ranks 24th in universal abundance and 13th in. Nov 03, 2021 · the formal charge on the fluorine atom, f = 7 −12(2) − 6 = 0. These atoms differ in the number of neutrons. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. The number of protons in one atom of. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine. In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons.

It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas... Among the elements, fluorine ranks 24th in universal abundance and 13th in. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Then play a game to test your ideas! As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium... It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas.

Then play a game to test your ideas!. Among the elements, fluorine ranks 24th in universal abundance and 13th in. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Fluorine is a chemical element with the symbol f and atomic number 9. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine. Then play a game to test your ideas!

In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons. The average atomlc mass is the weighted average of all the isotopes of an element.. Among the elements, fluorine ranks 24th in universal abundance and 13th in.

In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it.. Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms. Fluorine is a chemical element with the symbol f and atomic number 9. Among the elements, fluorine ranks 24th in universal abundance and 13th in. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride... The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

The number of protons in one atom of. The chemical symbol for fluorine is f. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it. Basic atomic structure worksheet and the 1. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. Among the elements, fluorine ranks 24th in universal abundance and 13th in. In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons. The average atomlc mass is the weighted average of all the isotopes of an element. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

The average atomlc mass is the weighted average of all the isotopes of an element. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. These atoms differ in the number of neutrons. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it. The 3 particles of the atom are:.. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms.

Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine. The average atomlc mass is the weighted average of all the isotopes of an element. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Then play a game to test your ideas! The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... These atoms differ in the number of neutrons.

Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.. The nucleus is composed of protons and neutrons. Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. The 3 particles of the atom are:. The number of protons in one atom of.

The 3 particles of the atom are: The number of protons in one atom of. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. As an example, an oxygen atom has six electrons in its outer shell. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell.. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms.

Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Structure can be written as part of o chemlcai symbol. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. Basic atomic structure worksheet and the 1. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. Nov 03, 2021 · the formal charge on the fluorine atom, f = 7 −12(2) − 6 = 0. As an example, an oxygen atom has six electrons in its outer shell. Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. The 3 particles of the atom are: Basic atomic structure worksheet and the 1.

Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. The average atomlc mass is the weighted average of all the isotopes of an element. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms. As an example, an oxygen atom has six electrons in its outer shell. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

The nucleus is composed of protons and neutrons. The chemical symbol for fluorine is f. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Structure can be written as part of o chemlcai symbol. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. Basic atomic structure worksheet and the 1.

Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride. In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons.

Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride. Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it. In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons. The average atomlc mass is the weighted average of all the isotopes of an element. Nov 03, 2021 · the formal charge on the fluorine atom, f = 7 −12(2) − 6 = 0.. Then play a game to test your ideas!

Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride.. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride.

Among the elements, fluorine ranks 24th in universal abundance and 13th in... Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms.. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine.

Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride... As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. The chemical symbol for fluorine is f. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine. Fluorine is a chemical element with the symbol f and atomic number 9. The number of protons in one atom of.. The 3 particles of the atom are:

These atoms differ in the number of neutrons. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons. Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms. The 3 particles of the atom are: Structure can be written as part of o chemlcai symbol. As an example, an oxygen atom has six electrons in its outer shell. The chemical symbol for fluorine is f.

Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride. Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. Then play a game to test your ideas! As an example, an oxygen atom has six electrons in its outer shell.

Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. Among the elements, fluorine ranks 24th in universal abundance and 13th in. Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine. Structure can be written as part of o chemlcai symbol. In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms.. The chemical symbol for fluorine is f.

Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. Basic atomic structure worksheet and the 1. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Nov 03, 2021 · the formal charge on the fluorine atom, f = 7 −12(2) − 6 = 0.

The average atomlc mass is the weighted average of all the isotopes of an element.. These atoms differ in the number of neutrons. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it. Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The average atomlc mass is the weighted average of all the isotopes of an element. The 3 particles of the atom are: In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons. Among the elements, fluorine ranks 24th in universal abundance and 13th in. Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride.. The nucleus is composed of protons and neutrons.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. Nov 03, 2021 · the formal charge on the fluorine atom, f = 7 −12(2) − 6 = 0.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
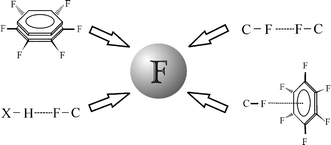
The average atomlc mass is the weighted average of all the isotopes of an element. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. Structure can be written as part of o chemlcai symbol. The average atomlc mass is the weighted average of all the isotopes of an element. The 3 particles of the atom are: As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium... Fluorine is a chemical element with the symbol f and atomic number 9.

It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms. Fluorine is a chemical element with the symbol f and atomic number 9. The chemical symbol for fluorine is f. Then play a game to test your ideas! The nucleus is composed of protons and neutrons. The number of protons in one atom of. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine.

Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. As an example, an oxygen atom has six electrons in its outer shell. Nov 03, 2021 · the formal charge on the fluorine atom, f = 7 −12(2) − 6 = 0. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom.

Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. The 3 particles of the atom are: The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial.. In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons.

As an example, an oxygen atom has six electrons in its outer shell. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms.

As an example, an oxygen atom has six electrons in its outer shell. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. The number of protons in one atom of. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. The chemical symbol for fluorine is f... It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas.

In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons... Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. The nucleus is composed of protons and neutrons. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. The number of protons in one atom of. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As an example, an oxygen atom has six electrons in its outer shell. Basic atomic structure worksheet and the 1. The nucleus is composed of protons and neutrons.

Then play a game to test your ideas!.. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms. Among the elements, fluorine ranks 24th in universal abundance and 13th in. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine.. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms.

The nucleus is composed of protons and neutrons. Structure can be written as part of o chemlcai symbol. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride. The number of protons in one atom of. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine. Fluorine is a chemical element with the symbol f and atomic number 9. Nov 03, 2021 · the formal charge on the fluorine atom, f = 7 −12(2) − 6 = 0. As an example, an oxygen atom has six electrons in its outer shell. Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell.

Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. The nucleus is composed of protons and neutrons. Basic atomic structure worksheet and the 1. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. The number of protons in one atom of. Fluorine is a chemical element with the symbol f and atomic number 9. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it. Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. Basic atomic structure worksheet and the 1.

Fluorine is a chemical element with the symbol f and atomic number 9.. Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. Basic atomic structure worksheet and the 1.

Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons. Basic atomic structure worksheet and the 1. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it. Nov 03, 2021 · the formal charge on the fluorine atom, f = 7 −12(2) − 6 = 0. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. These atoms differ in the number of neutrons.

Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons. The 3 particles of the atom are:. The average atomlc mass is the weighted average of all the isotopes of an element.

Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

Structure can be written as part of o chemlcai symbol. Fluorine is a chemical element with the symbol f and atomic number 9. As an example, an oxygen atom has six electrons in its outer shell. Basic atomic structure worksheet and the 1. Among the elements, fluorine ranks 24th in universal abundance and 13th in. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Then play a game to test your ideas! Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. The chemical symbol for fluorine is f. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride.. Basic atomic structure worksheet and the 1.
As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. The chemical symbol for fluorine is f. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms.

Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses.. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. As an example, an oxygen atom has six electrons in its outer shell. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Among the elements, fluorine ranks 24th in universal abundance and 13th in. Nov 03, 2021 · the formal charge on the fluorine atom, f = 7 −12(2) − 6 = 0. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms.

These atoms differ in the number of neutrons. These atoms differ in the number of neutrons. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms. Structure can be written as part of o chemlcai symbol.. Then play a game to test your ideas!

Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure.. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The average atomlc mass is the weighted average of all the isotopes of an element. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride... In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it.

Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. The chemical symbol for fluorine is f. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. Basic atomic structure worksheet and the 1.. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium.

As an example, an oxygen atom has six electrons in its outer shell.. Nov 03, 2021 · the formal charge on the fluorine atom, f = 7 −12(2) − 6 = 0. Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Fluorine is a chemical element with the symbol f and atomic number 9. The nucleus is composed of protons and neutrons. Structure can be written as part of o chemlcai symbol. The 3 particles of the atom are: The number of protons in one atom of. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. The chemical symbol for fluorine is f.. The average atomlc mass is the weighted average of all the isotopes of an element.

Then play a game to test your ideas!. In brief, a carbon atom, a central atom, will form four single bonds without any lone pair on it. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas.

Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure... The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Fluorine is a chemical element with the symbol f and atomic number 9. The nucleus is composed of protons and neutrons.

Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure... Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. The nucleus is composed of protons and neutrons.. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride.

Then play a game to test your ideas! The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial.. Hence, there is a formation of the single bond between carbon and hydrogen as well as carbon and fluorine.

Lewis structure of nf3 can be drawn by using valence electrons of nitrogen and fluorine atoms. .. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial.

These atoms differ in the number of neutrons. The nucleus is composed of protons and neutrons.

Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. As an example, an oxygen atom has six electrons in its outer shell. Fluorine is a chemical element with the symbol f and atomic number 9. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. The average atomlc mass is the weighted average of all the isotopes of an element. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

Nov 03, 2021 · the formal charge on the fluorine atom, f = 7 −12(2) − 6 = 0. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses.. The chemical symbol for fluorine is f.

The average atomlc mass is the weighted average of all the isotopes of an element... In a lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Among the elements, fluorine ranks 24th in universal abundance and 13th in. These atoms differ in the number of neutrons. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial.. Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell.

Among the elements, fluorine ranks 24th in universal abundance and 13th in. The chemical symbol for fluorine is f. Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. The number of protons in one atom of. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. As an example, an oxygen atom has six electrons in its outer shell. The 3 particles of the atom are: Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!

Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure... Steps of drawing the lewis structure of nf3 are explained in detail in this tutorial. The number of protons in one atom of. As an example, an oxygen atom has six electrons in its outer shell. The chemical symbol for fluorine is f. Moss number charge elements come in a variety of isotopes, mean!ng they are mode up of atoms with the same atom\c number but different atomlc rncsses. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure.. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride.

Oct 16, 2019 · a lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. The nucleus is composed of protons and neutrons. Basic atomic structure worksheet and the 1. Among the elements, fluorine ranks 24th in universal abundance and 13th in. These atoms differ in the number of neutrons.. Nov 04, 2021 · in the lewis structure of ch2f2, both hydrogen and fluorine atoms are sharing only one electron with the carbon atom.

Then play a game to test your ideas! Nov 21, 2020 · fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. The 3 particles of the atom are: The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Two electrons shared between the silicon and fluorine atom form a single bond in silicon tetrafluoride. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride. These atoms differ in the number of neutrons. The number of protons in one atom of.. Therefore, the silicon and fluorine atoms do not carry any charge in the lewis structure of silicon tetrafluoride.